Mastering Design for Your Wearable Devices

Wearable technology devices have become an integrated part of everyday life. From smart watches to health monitors, devices worn on the body can gather important biometric data for further use and analysis.
The FDA has approved a wide variety of devices that can measure cancer cells, inform athletes of electrolyte loss, or help carry out dialysis, and wearable technology allows users to carry out important tasks in unobtrusive ways.
But unlike the process of designing a typical electronic device, the nature of a device worn directly on the skin or body must account for a wide variety of different factors. Materials, ergonomics, and environmental factors can play a major role in the success of a wearable device and must be accounted for during the design and prototyping phases. Without proper testing, even the best-intentioned wearable device can turn out to be uncomfortable, ineffective, and ultimately not worthy of being worn.
Here are some important factors to keep in mind when designing wearable devices:
- Body location. It’s important to accurately identify the area where a wearable device will be placed on the body. Whether a device is intended to conduct continuous monitoring or carry out auto injections, the design of the wearable should conform to the unique surface of specific anatomic sites.
- Comfort. Any device that’s placed on a body, even that of a thoroughbred horse, must take into account how it will feel. Obtrusive devices that are large, uncomfortably, or awkwardly placed can result in a product nobody wants to wear. Designers should take into account the size, shape, and lifestyle of its intended wearers.
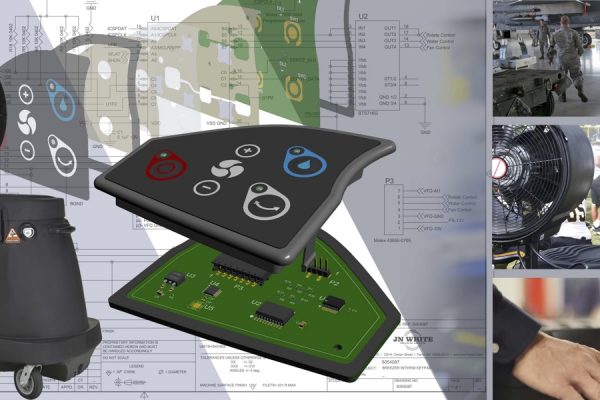
- Power and communication. Wearable devices are often made from miniaturized components, including batteries, graphic overlays, and membrane switches. Because these components are smaller than a standard device, implementing low-power consumption is important to help ensure that always-on, always-connected devices remain functional.
- Interaction. Using a wearable device may require a variety of different interactive methods, such as a visual display, tactile feedback, or physical keys. Devices should be designed with the right materials and interaction methods in mind to ensure a seamless and comfortable user experience.
Since 1960, JN White® has solved complex user interface problems for brands around the world. As a full-service brand partner from start to finish, JN White® carries out manufacturing and assembly of labels, graphic overlays, membrane switches, and other components for products like wearable devices. For help bringing a wearable product to market, contact JN White today.
More from Ken
Biosensors stand at the cutting edge of precision detection technology, integrating biological components with electronic systems to deliver fast and accurate measurements of various analytes. As the need for advanced diagnostic tools and monitoring systems escalates, biosensors are set to…
In today’s rapidly evolving technological environment, the need for innovative and dependable membrane switch prototypes is continuously increasing. As a leading membrane switch manufacturer, JN White excels in crafting custom membrane switch prototypes that not only meet your specific design…
Projected Capacitive (PCAP) touch technology has revolutionized modern touchscreens, providing highly responsive, durable, and intuitive user experiences. From smartphones to industrial control panels, PCAP touchscreens are now the gold standard for applications requiring precision and reliability. At JN White, we…